Abstract
Abstract
Background: Allogeneic hematopoietic stem cell transplantation (Allo-HSCT) is a potentially curative treatment in patients with acute myeloid leukemia (AML). The results of conventional BUCY regimen for high-risk acute myeloid leukemia (AML) are unsatisfactory because of higher relapse. Recent studies suggested survival advantage with idarubicin intensified BUCY regimen in Allo-HSCT for patients with high-risk AML.
Methods: This study was a multi-center, open, randomized-control study on the effects and safety of idarubicin 60mg/m2 combined with BUCY pretreatment program or BUCY pretreatment program on the overall survival (OS) rate and disease-free survival (DFS) rate of AML in high-risk group over a period of 2 years, where 118 high-risk AML patients underwent allogeneic peripheral blood stem cell transplantation (Allo-PBSCT) were enrolled between December 2012 and March 2017, from sibling or unrelated donors with well-matched human leukocyte antigens. This study was approved by the Medical Ethics Committee of the First Affiliated Hospital of Guangxi Medical University and was registered at the Chinese Bone Marrow Transplant Registry (CBMTR) and ClinicalTrials.gov (NCT 01766375). The conditioning regimen of experimental group (IDA-BUCY group, n=61) were: 1) Idarubicin 20mg/m2 given intravenously (IV) for 3 days (day -12 to day -10); 2) Busulphan (BU) 0.8mg/kg given intravenously (IV) four times per day for 4 days (day -7 to day -4); 3) cyclophosphamide (CTX) (60 mg/kg/day) given IV for 2 days (day -3 to day -2). The conditioning regimen of active comparator (BUCY group, n=57) were: 1) Busulphan (BU) 0.8mg/kg given intravenously (IV) four times per day for 4 days (day -7 to day -4); 2) cyclophosphamide (CTX) (60 mg/kg/day) given IV for 2 days (day -3 to day -2). GVHD prophylaxis of sibling Allo-PBSCT consisted of a combination of cyclosporin A (CSA), methotrexate(MTX) and mycophenolate mofetil(MMF) regimen [BMT 2009; 43(1):61-67]. Unrelated Allo-PBSCT used CSA, MMF, MTX, and anti-thymocytes globulin (ATG, 2.5 mg/kg/day given IV for 4 days,days -4 to day -1) for the prevention of GVHD.
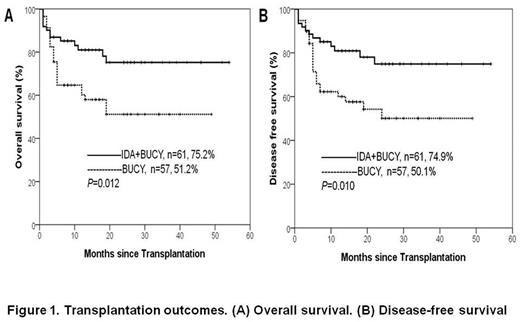
Results: The two groups were well balanced according to disease status, cytogenetic risk, and other variables. Data cut off for survival follow-up was June 30, 2017. The median follow-up time was 19 months (range, 4 months to 54 months). Six patients (9.8%) in the IDA+BUCY group and 15 (26.3%) in the BUCY group relapsed. Thirty-eight patients (IDA+BUCY group n=13, BUCY group n=25) died during the conduct of this study. The cumulative rates of transplant related mortality (TRM) of IDA+BUCY group and BUCY group were 12.7% (95% confidence interval [CI], 6.3 to 25.7) and 28.6% (95% CI, 16.8 to 51.7) (P=0.186), respectively. The cumulative rates of relapse was lower for the IDA+BUCY group (13.7%, 95% CI, 6.2 to 30.2) than for the BUCY group (28.1%, 95% CI, 17.8 to 44.3) (P=0.030). The OS and DFS of IDA+BUCY group at 2 years were 75.2% (95% CI, 63.0 to 87.4) and 74.9% (95% CI, 58.4 to 84.9), respectively. The OS and DFS of BUCY group at 2 years were 51.2% (95% CI, 36.5 to 65.9) and 50.1% (95% CI, 34.0 to 63.9), respectively. (Fig 1)
Conclusion: Our results demonstrate that Idarubicin intensified BUCY regimen could dramatically improve the survival of high-risk AML with low relapse and acceptable transplantation-related mortality. This strategy could be of great benefit for the treatment of patient with high-risk AML.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.
This icon denotes a clinically relevant abstract

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal